The Challenge
Healthy skin is synonymous with a healthy life, embodying confidence and self-awareness for those in good health, while also providing crucial information for individuals suffering from diseases. The mechanical properties of skin constitute a key indicator of its health state. However, up until now no measure exists to quantify the mechanics of skin health reliably. This leads to inferior patient treatment, unsuccessful drug developments and insufficient and non-personalized skin care procedures.
We, at Citus, enable doctors and skin care researchers to solve these challenges by measuring skin health through mechanical characterization. Citus is an ETH-spinoff and part of the Health Innovation Hub of University Hospital of Zürich (USZ).
Our Solution
Healthy skin shows distinct mechanical properties, determined primarily by the network of collagen fibres established by the embedded cells.
The endless cycle of synthesis and degradation of collagen ensures the integrity and full functionality of the tissue. However, aging processes as well as pathological conditions perturb this physiological turnover, leading to impairments of the skin functions, and, potentially, to severe consequences for patient's life.
Measuring the mechanical properties of skin on time and accurately hence becomes a turning point for the detection of these tissue changes.

At Citus we quantify these changes in an objective manner
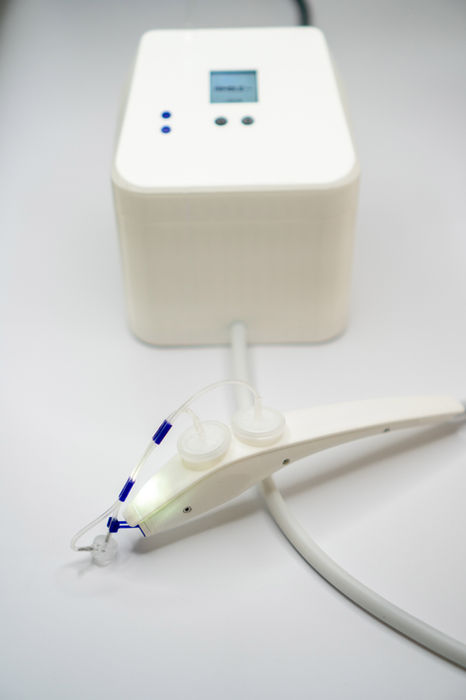
We introduce a novel ultra-light suction device, NIMBLEeva, overcoming the limits of the existing instruments. The specific working principle enables a lightweight design, preventing interaction between the observer and the suction probe, and by this the measurement outcome significantly improves.
Our goal is to support skin experts and clinicians in diagnosis, monitoring and control of skin conditions. NIMBLEeva offers a fast, pain-free, non-invasive measurement that can be directly performed on the patient and it is easy to use.
Skin Health Markets



COSMETIC
Challenge: Aging is most visible in the face, yet it is the most difficult area of skin assessment.
We enable: Personalized skin assessment and individual monitoring of aging in the face.
In detail: Efficacy testing in the cosmetic industry can encounter several challenges: • Subjectivity: Some efficacy measures, such as improvements of skin texture or overall appearance, can be subjective and vary from person to person. Ensuring consistency and reliability in subjective assessments can be challenging, particularly in consumer studies. • Standardization of Methods: Standardizing testing methods and protocols across different studies and laboratories is crucial for comparability and reproducibility of results. However, achieving consistency in testing procedures, equipment, and data analysis can be difficult, especially in multicenter or multi-institutional studies. • Cost and Time Constraints: Efficacy testing can be costly and time-consuming, particularly for complex studies involving clinical trials or long-term follow-ups. Balancing the need for comprehensive testing with budgetary and timeline constraints is a common challenge for cosmetic companies. Citus addresses all of these challenges with NIMBLEeva by + Objective skin characterization in facial areas for improved reliability in consumer studies + Standardized method for sensitive skin characterization + Fast skin assessment to an affordable price
MEDICAL
Challenge: Skin fibrosis assessment shows high variability.
We enable: Monitoring of changes in skin over time for tracking disease progression and adjusting treatment strategies accordingly.
Diagnosing skin fibrosis can present several challenges: • Clinical variability: Skin fibrosis can manifest in various ways, ranging from subtle changes in skin texture to more pronounced thickening and scarring. This variability in appearance can make diagnosis challenging, especially in the early stages when symptoms may be mild or nonspecific. • Inter-Observer Variability:nAssessment of disease severity often rely on visual inspection by trained evaluators, introducing inter-observer variability and subjective interpretation of findings. • Availability of Expertise: Diagnosing skin fibrosis may require expertise in dermatology, rheumatology, or other specialized fields. Access to healthcare providers with experience in diagnosing and managing fibrotic skin conditions may be limited, particularly in underserved areas or regions with fewer resources. • Progression over time: skin fibrosis can progress gradually over time, with symptoms worsening as the condition advances. Monitoring changes in skin texture, mobility, and functionality over time is important for tracking disease progression and adjusting treatment strategies accordingly. Citus addresses all of these challenges: + Reduce inter-observer variability by an easy-to-use and innovative design + Sensitive assessment of skin fibrosis in early stages + Easy-to-use and safe tool for assessment of fibrotic skin + Digital longitudinal monitoring of changes in skin characteristics
PHARMA
Challenge: Drug development suffers from missing non-invasive and objective clinical trial endpoints.
We enable: Better and more drugs to be approved, reduction of costs and risks.
In detail: Pharmaceutical companies encounter several challenges when testing new drugs for skin diseases: • Inter-Observer Variability: Assessment of disease severity often relies on visual inspection by trained evaluators, introducing inter-observer variability and subjective interpretation of findings. • Regulatory Requirements: Regulatory agencies impose stringent requirements for demonstrating the safety and efficacy of new drugs for skin diseases. To meet regulatory standards, objective outcome measures need to be implemented to prove the efficacy of drugs. • Patient Heterogeneity: Patients with skin diseases exhibit considerable heterogeneity in disease presentation, severity, and treatment responses. Stratifying patients based on disease subtypes, biomarkers or genetic factors can improve trial outcomes. • Invasive or Time-Consuming Procedures: Some objective measures of disease severity, such as skin biopsies or imaging studies, are invasive, costly or time-consuming to perform. These procedures can pose logistical challenges in clinical trial settings and may not be feasible for routine monitoring of disease progression or treatment response. Citus adresses these challenges with the development of novel objective measures that + Reduce inter-observer variability by an easy-to-use and innovative design + Support patient selection to improve trial outcome + Provide non-invasive, fast and reliable outcome measures of skin characteristic